The world of radioactivity can be a complex and intimidating place, but understanding its effects over time is crucial for a variety of fields, including medicine, energy, and environmental science. In this article, we'll delve into the world of radioactivity and explore its effects over time, using diagrams to help illustrate key concepts.
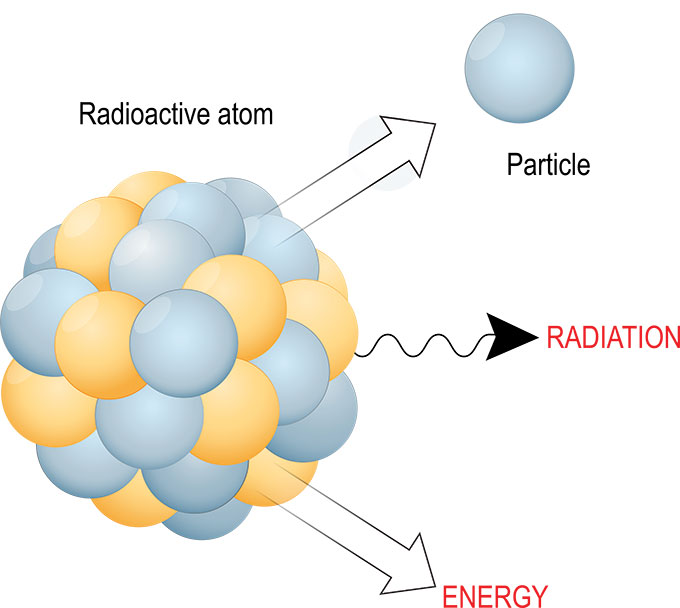
Radioactivity is a natural process that occurs when an atom's nucleus decays, releasing energy in the form of radiation. This process can occur spontaneously, or it can be triggered by external factors, such as exposure to radiation. Radioactivity is all around us, from the cosmic rays that bombard the Earth from space to the radioactive isotopes that are present in the ground and in our own bodies.
Understanding Radioactive Decay

Radioactive decay is the process by which unstable atoms lose energy and stability. This process occurs when an atom's nucleus is unstable, meaning it has too many or too few neutrons compared to protons. As the nucleus decays, it releases radiation in the form of alpha, beta, or gamma rays.
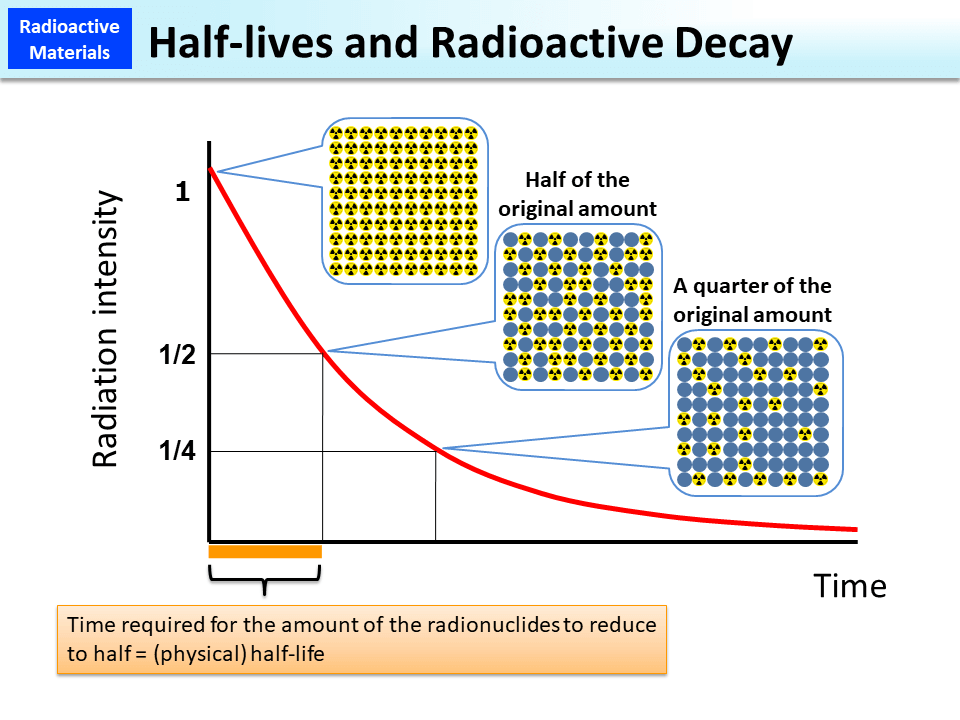
The rate of radioactive decay is measured in terms of half-life, which is the time it takes for half of the unstable atoms in a sample to decay. Half-life can range from a fraction of a second to billions of years, depending on the specific isotope.
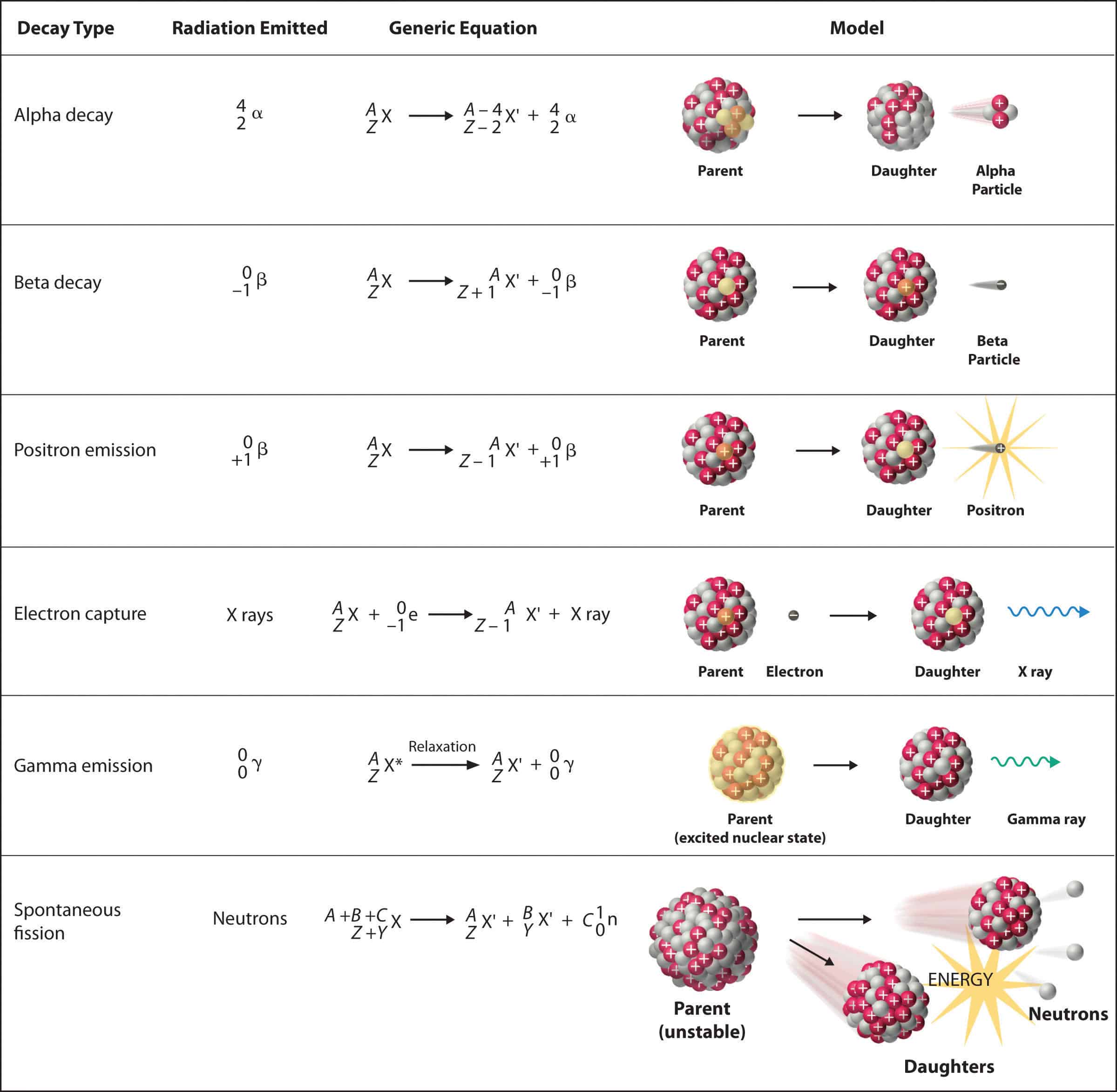
Types of Radioactive Decay
There are several types of radioactive decay, including:
Alpha decay: This type of decay occurs when an atom releases an alpha particle, which consists of two protons and two neutrons. Alpha decay is the most common type of decay and is often seen in heavy elements, such as uranium and thorium. Beta decay: This type of decay occurs when an atom releases a beta particle, which is either a positron (the antiparticle of an electron) or an electron. Beta decay can occur in two forms: beta minus (β-) decay, in which a neutron is converted into a proton and an electron, and beta plus (β+) decay, in which a proton is converted into a neutron and a positron. Gamma decay: This type of decay occurs when an atom releases a gamma ray, which is a high-energy photon. Gamma decay often occurs in conjunction with alpha or beta decay.
Effects of Radioactivity on the Environment

Radioactivity can have a significant impact on the environment, particularly when it comes to human exposure. Radioactive isotopes can contaminate soil, water, and air, leading to a range of health problems.
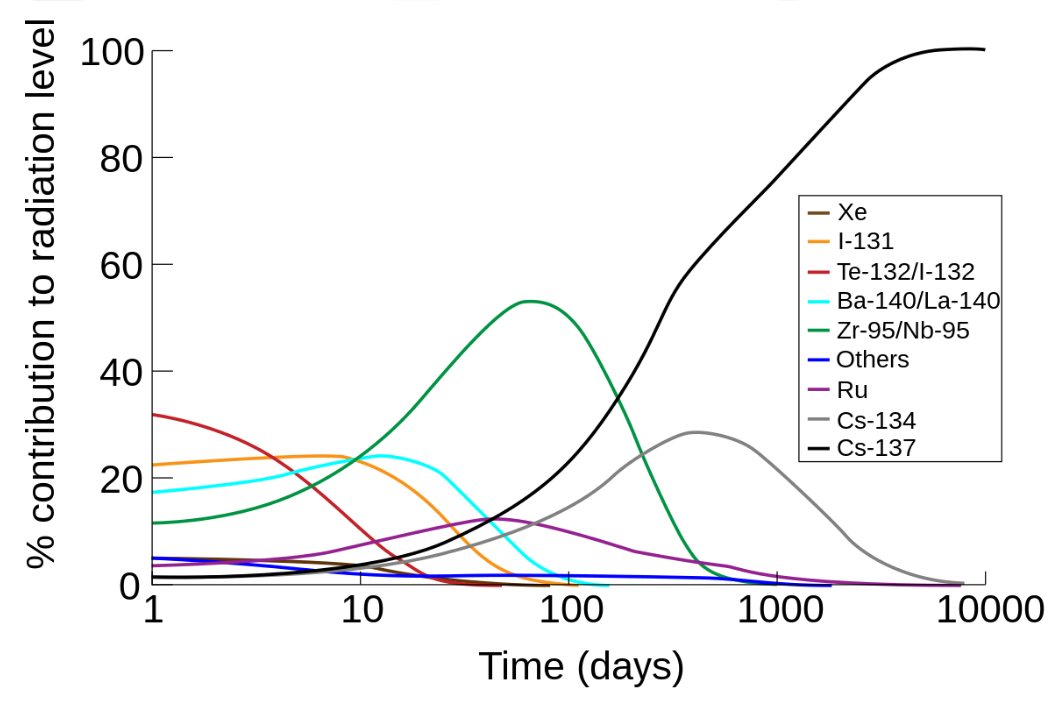
One of the most significant environmental impacts of radioactivity is the creation of radioactive waste. This waste can remain hazardous for thousands of years, posing a significant risk to human health and the environment.
Environmental Impacts of Radioactive Waste
Radioactive waste can have a range of environmental impacts, including:
Soil contamination: Radioactive isotopes can leach into the soil, contaminating crops and water sources. Water pollution: Radioactive isotopes can contaminate water sources, posing a risk to human health and aquatic ecosystems. Air pollution: Radioactive isotopes can release radioactive particles into the air, posing a risk to human health and the environment.
Effects of Radioactivity on Human Health

Radioactivity can have a range of health effects on humans, particularly when it comes to exposure to high levels of radiation. Some of the most significant health effects of radioactivity include:
Cancer: Exposure to high levels of radiation can increase the risk of cancer, particularly leukemia and other blood cancers. Genetic damage: Radiation can cause genetic damage, leading to birth defects and other health problems. Radiation sickness: High levels of radiation can cause radiation sickness, which can lead to a range of symptoms, including nausea, vomiting, and even death.
Health Effects of Radioactive Exposure
The health effects of radioactive exposure can vary depending on the level and duration of exposure. Some of the most significant health effects of radioactive exposure include:
Low-level exposure: Low-level exposure to radiation can increase the risk of cancer and genetic damage. High-level exposure: High-level exposure to radiation can cause radiation sickness and even death.
Radioactivity in Medicine

Radioactivity has a range of applications in medicine, particularly in the diagnosis and treatment of cancer. Some of the most significant medical applications of radioactivity include:
Cancer treatment: Radioactivity can be used to treat cancer, particularly in the form of radiation therapy. Imaging: Radioactivity can be used in medical imaging, particularly in the form of positron emission tomography (PET) scans.
Medical Applications of Radioactivity
Some of the most significant medical applications of radioactivity include:
Cancer treatment: Radioactivity can be used to treat cancer, particularly in the form of radiation therapy. Imaging: Radioactivity can be used in medical imaging, particularly in the form of PET scans.
Conclusion: Understanding Radioactivity
Radioactivity is a complex and fascinating topic that has a range of applications in fields such as medicine, energy, and environmental science. Understanding radioactivity is crucial for a variety of reasons, including the need to mitigate the environmental impacts of radioactive waste and the need to harness the medical applications of radioactivity.
By exploring the effects of radioactivity over time, we can gain a deeper understanding of this complex topic and its many applications.
What is radioactivity?
+Radioactivity is a natural process that occurs when an atom's nucleus decays, releasing energy in the form of radiation.
What are the health effects of radioactivity?
+The health effects of radioactivity can vary depending on the level and duration of exposure, but can include cancer, genetic damage, and radiation sickness.
What are the environmental impacts of radioactivity?
+The environmental impacts of radioactivity can include soil contamination, water pollution, and air pollution.
We hope this article has provided a comprehensive overview of radioactivity and its effects over time. Whether you're a scientist, a medical professional, or simply someone interested in learning more about this complex topic, we encourage you to continue exploring the world of radioactivity.
Gallery of Radioactivity Effects Over Time Explained In Diagrams